Empirical Formula Calculator
Quickly calculate the simplest ratio of elements in a compound using mass or percentage data.
How the Empirical Formula Calculator Works
Our Calculator converts the values you enter into mole-based ratios using accurate atomic masses, then simplifies those ratios to the smallest whole numbers to determine the compound’s simplest chemical composition. It follows standard chemistry rules taught in classrooms and laboratories, updating results instantly as inputs change while clearly displaying each calculation step so users can understand how the final formula is formed.
Choose Input Type
Select whether you want to enter values by mass (grams) or by percentage composition based on the data you have.
Add Elements
Choose each element using its chemical symbol and enter the corresponding value. You can add or remove elements as needed.
View the Result
The calculator instantly converts values into mole ratios, simplifies them, and displays the final empirical formula with clear calculation steps.
Table of Contents
What Is an Empirical Formula?
An empirical formula describes the simplest whole-number ratio of atoms present in a chemical compound. Instead of showing the exact number of atoms, it focuses on how elements relate to each other proportionally. This makes it especially useful when the full molecular structure is unknown.
For example, a compound may contain multiple atoms of carbon, hydrogen, and oxygen, but the empirical formula reduces that information to its most basic ratio. This simplified representation allows chemists to quickly understand elemental relationships without needing detailed molecular data.
Empirical Formula vs Molecular Formula
The molecular formula is always a whole-number multiple of the empirical formula. This relationship connects simplified ratios with real molecular data, allowing chemists to move from basic composition to complete structural understanding.
Empirical Formula
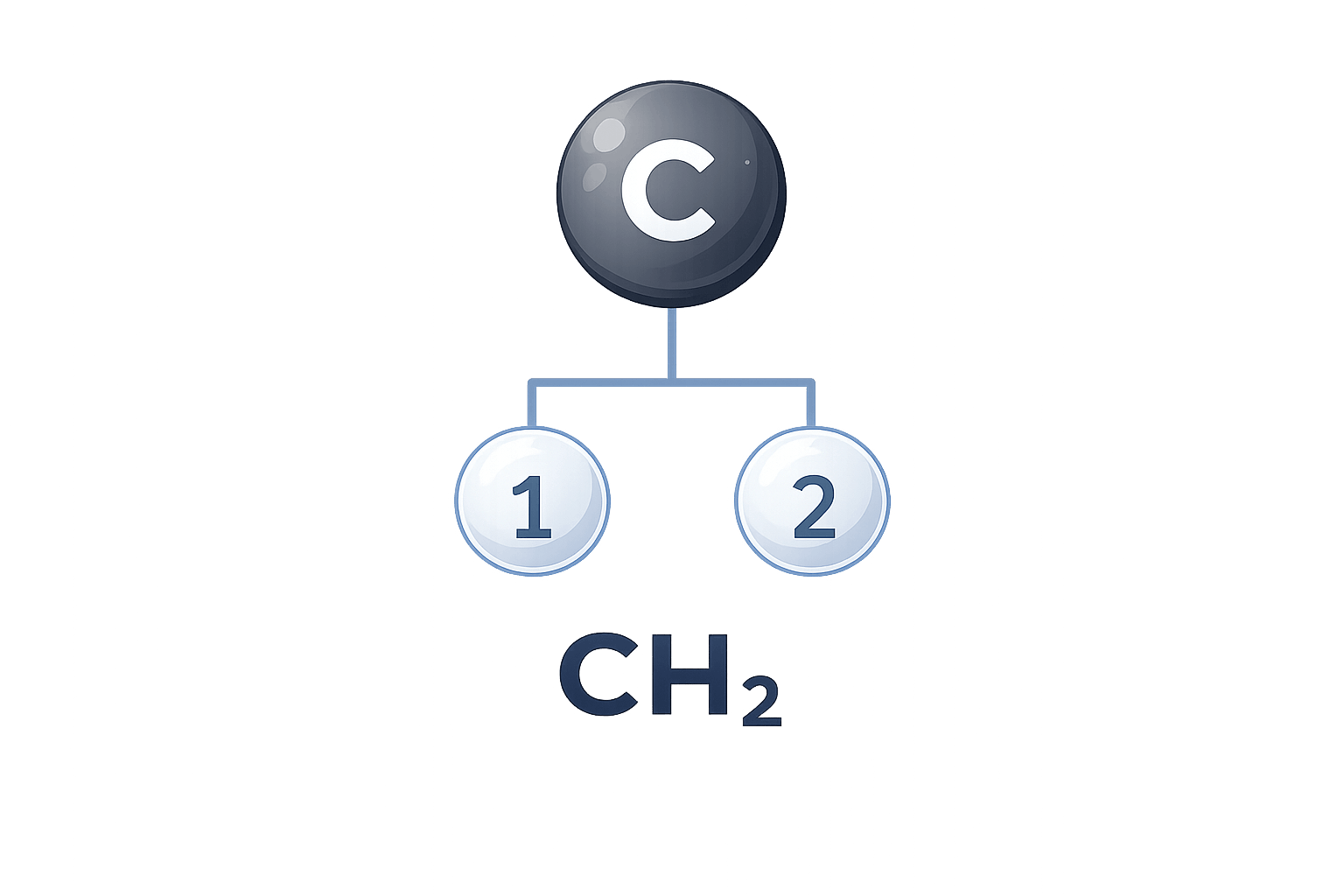
The empirical formula shows the simplest whole-number ratio of elements present in a compound. It focuses on proportions rather than the actual number of atoms. This makes it useful when working with composition data, such as mass or percentage values, where the exact molecular structure is not yet known.
Chemists often calculate the empirical formula first because it provides a clear starting point for understanding how elements combine. It is commonly used in classroom problems, lab experiments, and introductory chemistry calculations.
Key points:
- Represents the simplest ratio of elements
- Does not show the true number of atoms
- Derived from mass or percentage composition
- Used as the first step in formula calculations
Molecular Formula
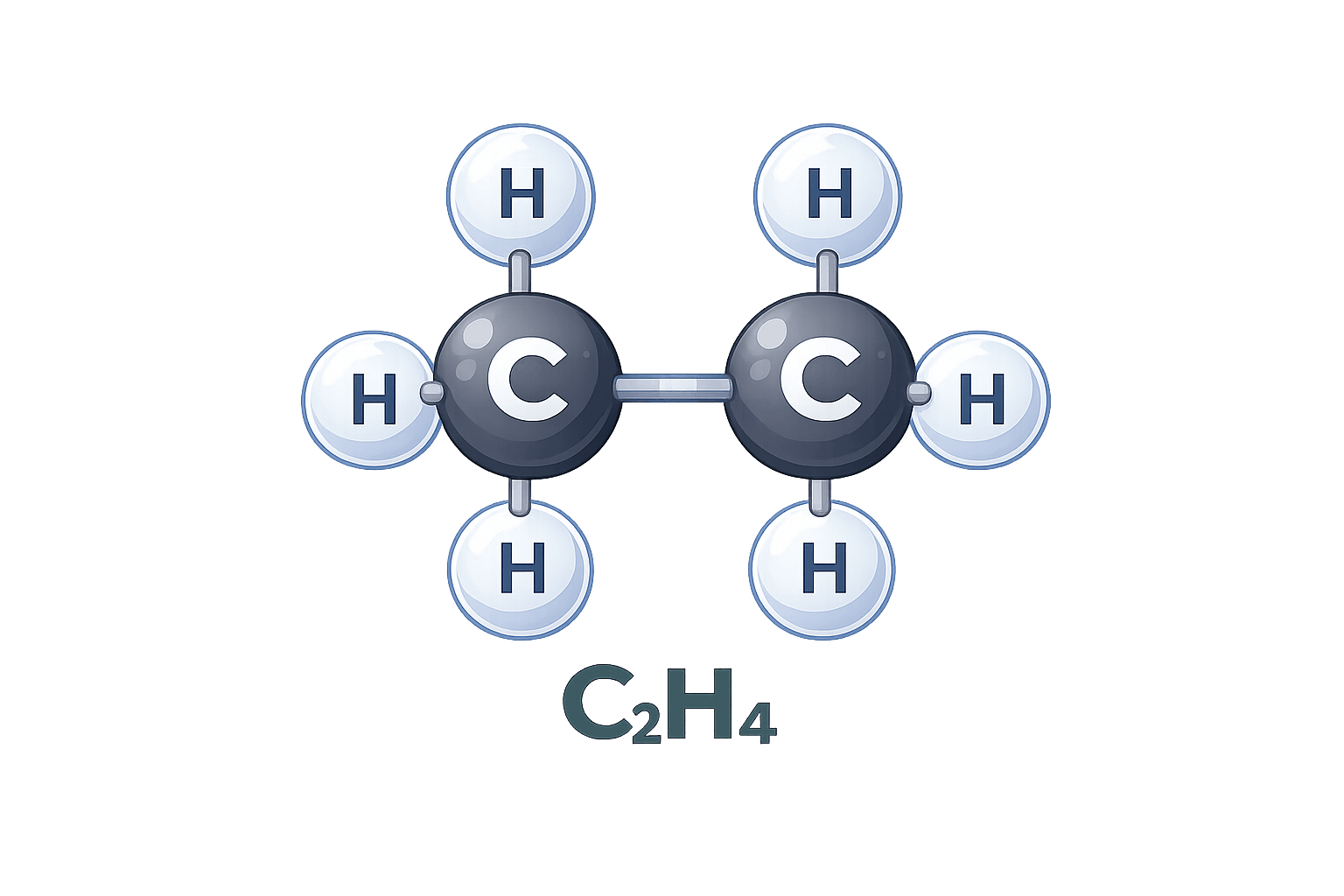
The molecular formula shows the actual number of atoms of each element in a compound. It provides a complete picture of the molecule’s composition and is typically determined after the empirical formula is known.
To find the molecular formula, the molar mass of the compound is compared with the mass of the empirical formula. This step reveals how many times the empirical formula must be multiplied to match the real molecular structure, which is why molecular formulas are often larger and more detailed.
Key points:
- Shows the exact number of atoms
- Based on molar mass information
- Often a multiple of the empirical formula
- Used to describe the full molecular structure
How to Calculate an Empirical Formula Manually
To understand how to calculate empirical formula values by hand, the process begins by converting given data into moles. This may involve turning masses or percentages into gram values and then dividing by each element’s molar mass.
After converting to moles, each value is divided by the smallest mole quantity to form ratios. These ratios are then adjusted until whole numbers are obtained. While the steps are straightforward, small calculation mistakes can lead to incorrect results, which is why many learners use calculators to verify their answers.
You can also watch this YouTube video if you want to calculate it manually.
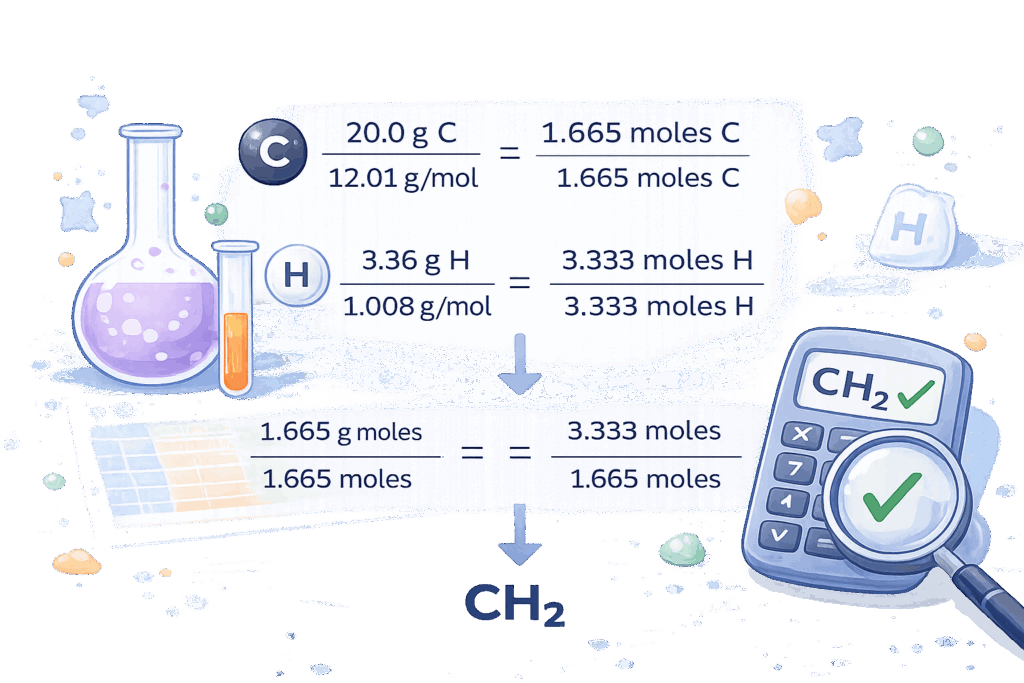
Finding Empirical Formula from Percent Composition
One of the most common chemistry problems involves determining a formula using percentage data. To solve this, chemists assume a total sample mass, usually 100 grams, which allows percentages to be treated as grams directly.
From there, the mass of each element is converted into moles, ratios are formed, and the simplest whole numbers are identified. This method is essential when learning how to find empirical formula from percent composition or how to find empirical formula from percent values, especially in lab-based or exam-style questions.
Common Mistakes to Avoid
A frequent mistake is rounding mole ratios too early, which can distort the final formula. Another issue is confusing empirical formulas with molecular formulas, particularly when molar mass data is involved.
Some learners also forget to simplify ratios fully or misinterpret percentage values when converting them into grams. These small errors can significantly affect results, making it important to follow each step carefully or verify answers using a reliable calculator.
Frequently Asked Questions
Find clear answers to common questions about empirical formulas, calculations, and chemistry concepts explained in a simple way.
What is an empirical formula in chemistry?
An empirical formula shows the simplest whole-number ratio of elements present in a compound, not the actual number of atoms.
How is an empirical formula different from a molecular formula?
An empirical formula gives the simplest ratio of elements, while a molecular formula shows the exact number of atoms in a molecule.
What data do I need to calculate an empirical formula?
You need either the mass of each element or the percentage composition of the compound.
Can an empirical formula and molecular formula be the same?
Yes, if the simplest ratio already represents the actual number of atoms, both formulas will be identical.
Why do empirical formula calculations use mole ratios?
Mole ratios allow elements to be compared on a particle level, which is necessary to find correct chemical proportions.
How do you calculate an empirical formula from percent composition?
Percent values are converted to grams, then to moles, and finally simplified into the smallest whole-number ratio.
What causes large numbers in an empirical formula?
Large subscripts appear when mole ratios cannot be simplified further into smaller whole numbers.
Is it necessary to show calculation steps?
Showing steps helps verify accuracy and makes it easier to understand where mistakes may occur.
Can this calculator be used for school and exams?
Yes, our Empirical Formula Calculator is useful for homework, practice problems, lab work, and exam preparation.
Why should I verify manual calculations with a calculator?
Manual calculations can include rounding or arithmetic errors, so using a calculator helps confirm correct results.
Some of our popular calculators: